A novel cysteine knot protein of sperm motility initiating
substance revealed a significant process of diversification of reproductive
modes to establish internal fertilization in amphibians. (Press release; Oct 18th
/2016)
Establishment
of internal fertilization in amphibians would have greatly contributed to the
transition of vertebrates from water to land, which is suggested by the fact
that extant terrestrial vertebrates commonly undergo internal fertilization for
their natural reproduction. Whereas, developing reproductive technologies allow
reproduction of domestic animals or human by artificial external fertilization.
However, little is known about the mechanism involving in the diversification
of reproductive modes to establish internal fertilization in vertebrates. Yokoe et al in PLOS ONE 11: e0160445 (2016) first addresses
that mechanism by identifying the gene encoding a novel cysteine knot protein
of sperm motility initiating substance (SMIS), which is a key protein for the
success of internal fertilization of the newt Cynops pyrrhogaster. The SMIS is an amphibian-specific gene since no analogous gene was
found in the gene data of any model organisms. Bioassay using an active site
peptide of the SMIS demonstrated that SMIS was a common enhancer of sperm
motility in anurans and urodeles, and enabled sperm
of C. pyrrhogaster
and the tree frog Rhacophorus arboreus to
penetrate egg coat matrix highly specialized in physicochemical feature for the
internal fertilization and for the arboreal fertilization, respectively. Sperm
of each species is known to show unique motion based on its specific morphology
(long and stiff tail with a undulating membrane in C. pyrrhogaster and spiral shape in R. arboreus),
which can provide directional motility only in the viscous matrix of the egg
coat. These facts suggest the evolutionary history that sperm motility and
morphology has been selectively adapted to the egg coat matrix specialized for
the specific reproductive mode in the diversification of reproductive modes of
amphibians, and the evolution of SMIS gene might facilitate the selective
alteration of sperm motility and morphology to finally establish internal
fertilization.
Internal fertilization-specific mechanism of
initiation of sperm motility in amphibians: Its molecular basis and evolution
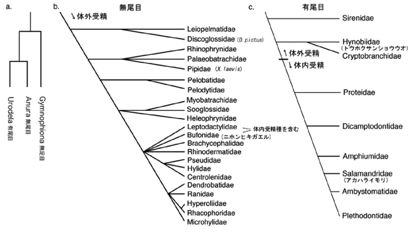
Reproductive modes of amphibians
are highly diversified and, along the evolutional process, internal
fertilization is suggested to be established from
fundamental external fertilization (Duellman and Trueb. 1986). Actually, 90% of urodele
amphibians undergo external fertilization, which occurs in female cloaca. The
aim of our project is to understand how the internal fertilization of
amphibians is established, which should be a critical event in their adaptation
to a variety of conditions on land.
Amphibian eggs are surrounded by jelly layer, thick
extracellular matrices secreted and accumulated in the oviduct. Multiple
works show significance of the jelly layer for the success of amphibian fertilization
in both external and internal conditions. We have found a new correlation of
sperm-egg interaction specific for the urodele mode
of internal fertilization in the jelly layer of a red-bellied newt, Cynops pyrrhogaster
(Ukita et al., 1999; Watanabe and Onitake,
2003).
It is based on the specific
localizations of acrosome reaction-inducing substance and sperm
motility-initiating substance on the jelly surface (Watanabe et al., 2009;
Watanabe et al., 2010), which cause a unique pattern of motility initiation
among sperm quiescently stored in sperm reservoir for long time. Our recent
research focuses on 1) the molecular basis of the novel mechanism of sperm
motility initiation and 2) its establishing history on amphibian evolution.

Duellman WE, Trueb
L. 1994. Biology of amphibians. Baltimore: Johns Hopkins University
Press.
Watanabe A, Onitake K. 2002. Zool. Sci. 19:
1341-1347.
Watanabe A, Fukutomi K, Kubo H, Ohta
M, Takayama-Watanabe E, Onitake
K. 2009. Mol. Reprod. Dev.79: 399-406.
Watanabe T, Kubo H, Takeshima S, Nakagawa M, Ohta M, Kamimura S, Takayama-Watanabe E, Watanabe A, Onitake
K. 2010. Int. J. Dev. Biol. 54: 591-597.